October 29, 2024
3 min read
This Sponge Captures the Teeny Bits of Gold in Electronic Waste
A self-building sponge that efficiently collects gold could eliminate some harsh methods used to process e-waste

If all 62 million metric tons of electronic waste produced in a year were loaded into garbage trucks, they’d encircle the planet bumper to bumper, according to a recent United Nations report. And hidden in that monstrous traffic jam would be startling amounts of precious metals, including gold—crucial in electronics because it conducts electricity, stretches into wires and won’t easily corrode. Modern iPhones have gold in their cameras, circuit boards and USB-C connector. Pound for pound, there is more gold in cell phones than in ore from a typical gold mine.
But wringing precious metals from trashed electronics is a harsh business. Using energy-intensive smelters, recycling facilities process e-waste with punishing heat. Or they’ll use caustic agents to break down bulk electronics into liquids full of metal ions, an approach that then requires complex electrochemical processes and toxic treatments to extract valuable elements in their metallic forms. The search for environmentally friendlier methods that skip those additional steps has led materials scientists down some unusual alleyways: For instance, an aerogel made from whey protein—a cheese by-product—can capture gold ions from computer motherboards bathed in acid. Another experimental material, described this month in the Proceedings of the National Academy of Sciences USA, combines graphene (a one-atom-thick sheet of carbon) with chitosan, a sugar found in shrimp shells. Because chitosan spontaneously attaches to the carbon sheet, the sponge essentially builds itself.
In their initial experiments, the study authors used the sponge to filter water that contained gold ions. The pale yellow liquid became clear as gold particles piled up on the graphene’s surface. Once there, the ions reacted with chitosan, a natural reducing agent that helped transform the gold back into its metallic form. The researchers also tested the sponge on partially processed e-waste; when the scientists increased the liquid’s acidity to a pH of 3, the chitosan in the sponge captured the remaining gold while ignoring other metals. Although it requires an acidic environment, the sponge could eliminate the need for further processing, which can rely on poisons such as cyanide to obtain metallic gold from liquids. “Our method allows for efficient recovery of gold directly from the waste mixture,” say study co-authors Daria Andreeva-Baeumler and Konstantin Novoselov, materials scientists at the National University of Singapore.
On supporting science journalism
If you’re enjoying this article, consider supporting our award-winning journalism by subscribing. By purchasing a subscription you are helping to ensure the future of impactful stories about the discoveries and ideas shaping our world today.
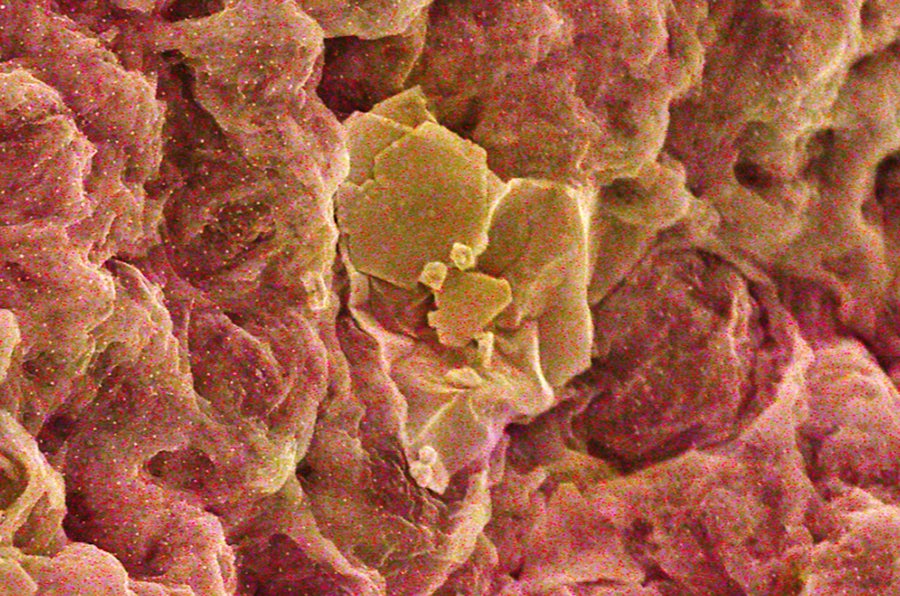
An electron microscope image of gold particles (yellow) on the graphene-chitosan sponge.
The new material is one of the most potent gold adsorbers ever created. (Adsorption is similar to the more familiar absorption, but adsorbed things accumulate on surfaces while absorbed things are internalized. It’s the difference between a mustard glob on your chin and the hot dog you just ate.) The sponge collected up to 99.5 percent of the gold by weight from liquids with gold concentrations as low as three parts per million.
“To the best of my knowledge, this is a record high value,” says Swiss Federal Institute of Technology Zurich physicist Raffaele Mezzenga, an author of the whey protein aerogel study, who wasn’t involved with the research on the graphene-chitosan combo. He notes that as efficient as the sponge is, the components needed to make it aren’t cheap, and he questions whether it would be a workable option “under real operating conditions.” Adapting the technique for industrial-scale use, Novoselov and Andreeva-Baeumler say, “is indeed the next step in our research.”









